Therapeutic Hyaluronic Acid and Peptide Composition for Regenerative Repair of Cartilage Injury
Innovative disease-modifying method to treat knee cartilage injuries and prevent development of post-traumatic osteoarthritis (PTOA)
Technology
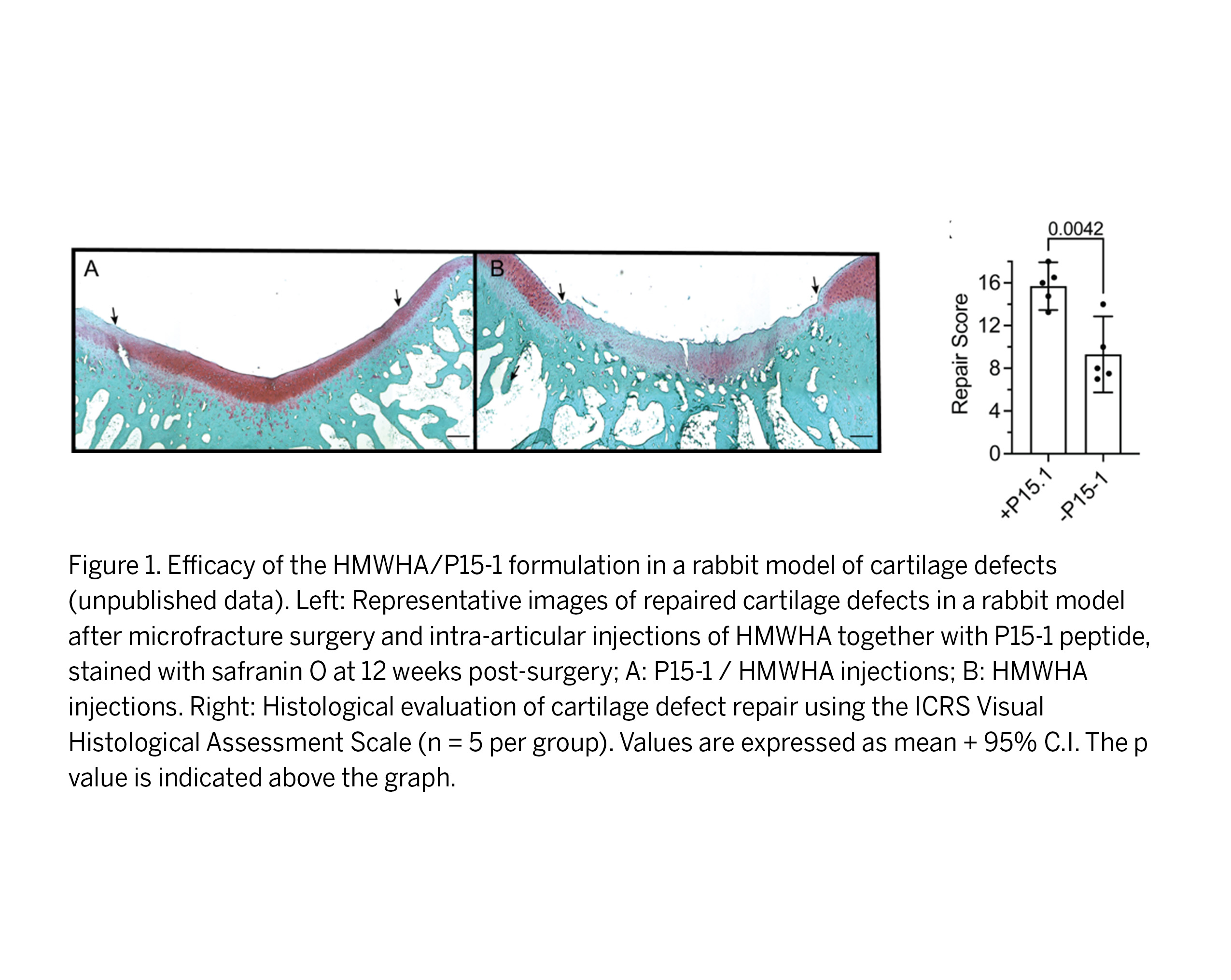
This invention pertains to the development of a novel therapeutic composition comprised of high molecular weight hyaluronic acid (HMWHA) and a 15-amino acid peptide (termed “P15-1”) for the treatment of cartilage injuries and the prevention of PTOA. HMWHA is an FDA-approved treatment to alleviate pain and restore functionality in patients afflicted with osteoarthritis. The peptide P15-1 was engineered to bind hyaluronic acid (HA) while competitively reducing the binding of HA to the Receptor for Hyaluronan Mediated Motility (RHAMM). The P15-1 peptide and HMWHA work synergistically to block the stimulation of downstream pro-inflammatory and fibrotic responses to joint injury. As shown in published proof-of-concept studies (see list below), the HMWHA/P15-1 formulation was found to modulate both the number and activity of multiple cell types needed for cartilage repair, providing an anti-inflammatory and reparative environment for tissue regeneration. Specifically, this formulation was shown to facilitate the formation of native hyaline repair cartilage (as found in healthy joints) and prevent unwanted fibrocartilage tissue that results from current repair procedures. Moreover, in recent unpublished studies using rabbit models of cartilage defects, the HMWHA/P15-1 formulation was found to significantly improve cartilage regeneration relative to HMWHA alone (see Figure 1). Additionally, in a rat knee injury model (Ruiz et al. Osteoarthritis Cartilage 2022), the P15-1 peptide showed higher retention in the injury site after injection than a scrambled control peptide, demonstrating the site-specific selectivity of P15-1. Altogether, these POC findings establish the HMWHA/P15-1 composition as a promising therapeutic candidate to improve cartilage regeneration and ultimately prevent PTOA development after joint injuries.
Background
Knee cartilage injuries affect approximately 900,000 Americans annually and lead to significant pain and morbidity. For such injuries, more than 200,000 surgical procedures are performed each year, yet in more than 50% of patients, these treatments lead to the formation of poorly functioning fibrotic articular cartilage, early-onset PTOA, and eventual knee replacement. Initial success rates for conventional microfracture surgery or autologous chondrocyte implantation (standard of care treatments for cartilage injuries) are only approximately 65% and 55%, respectively. Among these patients, 48% and 57%, respectively, develop PTOA within 15 years. Additionally, these current methods present important limitations: (i) high cost; (ii) only applicable to small cartilage defects; and (iii) are not curative treatments. Therefore, there is an unmet medical need for innovative disease-modifying therapeutic strategies to improve cartilage regeneration after injuries and mitigate the progression of PTOA.
Development Status
The inventors have demonstrated robust pre-clinical POC for the HMWHA/P15-1 formulation. The next step is to test the formulation for safety and efficacy in higher-order organisms, specifically, PTOA mini pig models.
Applications
- Treatment of joint injuries, including damaged menisci, cartilage defects, and cartilage lesions
- Prevention of PTOA
Advantages
- Disease modifying-activity: Stimulates the formation of native hyaline repair cartilage for expected prevention of PTOA development
- Restorative mechanism of action: Inhibition of the HA-RHAMM interaction with P15-1 restores normal anti-inflammatory HA-CD44 signaling
- Favorable target expression profile: RHAMM is expressed at low levels in homeostatic conditions but is upregulated in response to stress and injury
- Improves upon an FDA-approved therapeutic: Leverages the analgesic and viscoelastic properties of clinically-approved hyaluronic acid
- Targeted therapy: The composition can be specifically targeted and retained at the site of injury thereby minimizing the risk of off-target effects
- Alternative to invasive and costly surgery: The composition is relatively inexpensive and could be injected immediately after injury
Intellectual Property
A U.S. non-provisional patent has issued covering the composition and method of use of this therapeutic composition (US10449229B2).
-
expand_more mode_edit Authors (3)Mary Cowman, PhDThorsten Kirsch, PhDEric Strauss, MD
-
expand_more library_books References (3)
- Kirsch T, Zhang F, Braender-Carr O, Cowman MK (2021), Protective Effects of a Hyaluronan-Binding Peptide (P15-1) on Mesenchymal Stem Cells in an Inflammatory Environment
- Shortt C, Luyt LG, Turley EA, Cowman MK, Kirsch T. A (2020), A Hyaluronan-binding Peptide (P15-1) Reduces Inflammatory and Catabolic Events in IL-1β-treated Human Articular Chondrocytes
- Ruiz A, Duarte A, Bravo D, Ramos Gavilá E, Zhang C, Cowman MK, Kirsch T, Milne M, Luyt LG, Raya JG (2022), In vivo multimodal imaging of hyaluronan-mediated inflammatory response in articular cartilage
-
expand_more cloud_download Supporting documents (2)Product brochureTherapeutic Hyaluronic Acid and Peptide Composition for Regenerative Repair of Cartilage Injury.pdfMarketing BriefNYU - Therapeutic Hyaluronan and Peptide Composition - Marketing Brief -COW02-02.pdf (110 KB)