Targeting mutant NT5C2 in Acute Lymphoblastic Leukemia (ALL)
Unmet Need
Development of a targeted therapy through the identification of small molecular inhibitors to treat and prevent relapsed Acute Lymphoblastic Leukemia (ALL) in patients harboring NT5C2 mutations.
Technology
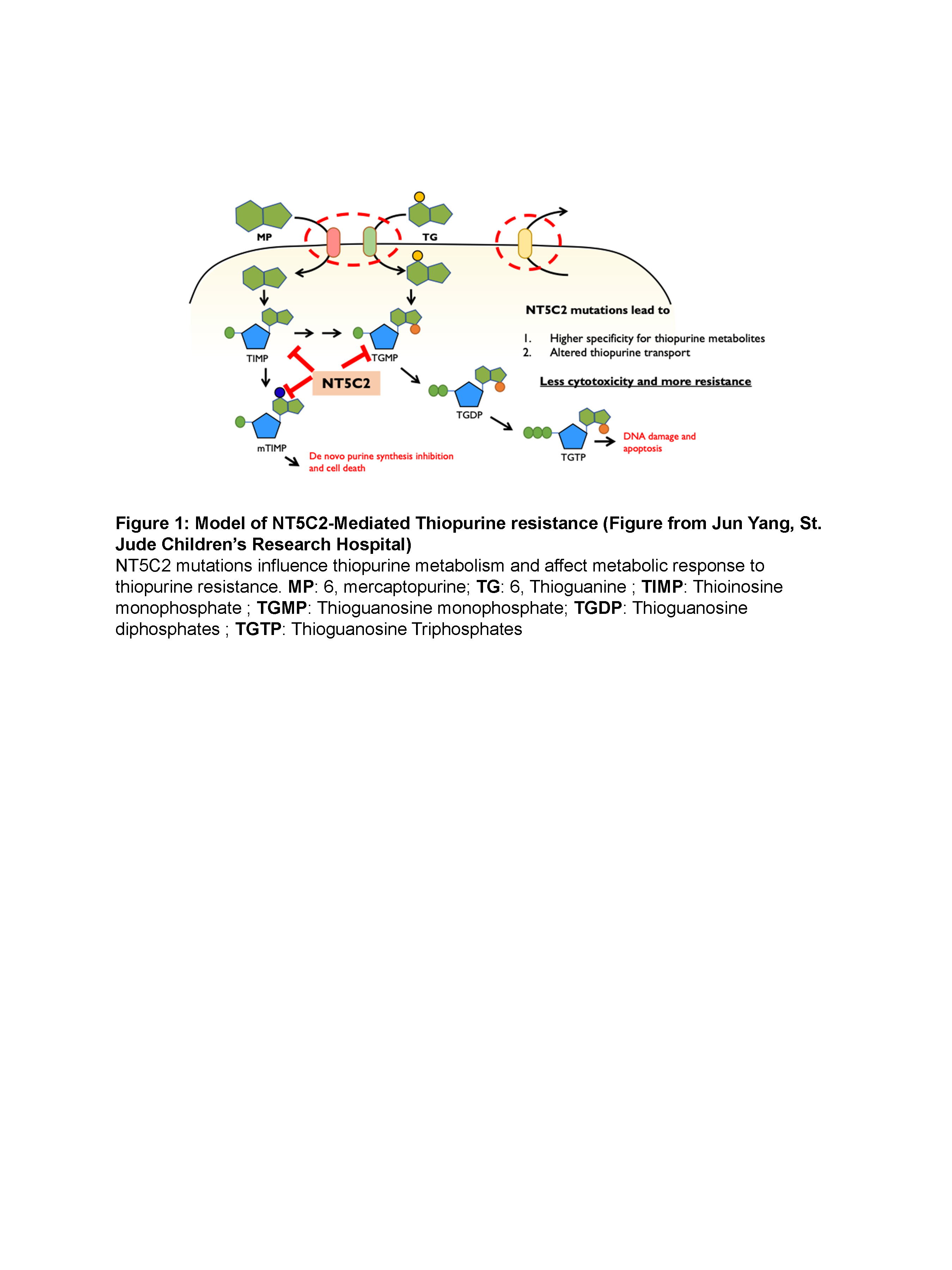
The Carroll Lab has identified gain of function mutations in the gene encoding for the NT5C2 enzyme, in relapsed specimens from pediatric patients with ALL. Cell-based experiments have shown that induced expression of the mutant form of NT5C2 in ALL cells confers chemoresistance to purine nucleoside analogs. A library HTS assay (240K compounds) followed by SAR-by-Catalog was conducted in order to identify small molecule inhibitors targeting the activity of the mutant form of the NT5C2 enzyme. This resulted in the identification of two lead compounds, with chemotype A presenting excellent inhibitory activity, in the low micro - high nano range. This has potential as a future therapeutic that could be administered prophylactically after first-line chemotherapy but before clinical relapse presents itself. Moreover, the target patient population could be extended to other blood cancer types treated with thiopurines.
Background
Acute lymphoblastic leukemia (ALL) is the most common childhood cancer. Relapse carries a poor prognosis and is observed in approximately 15% of pediatric patients after receiving chemotherapy, and most succumb to the disease. Mutations of the gene coding for the NT5C2 enzyme, a 5’-nucleotidase, have been identified in relapse specimens from patients as a main driver of chemoresistance. The NT5C2 mutants exhibit a significantly increased enzymatic activity that confer resistance to thiopurines, an essential component of chemotherapy used to treat ALL (figure 1). Moreover, patients harboring NT5C2 mutations relapse early and have the worst prognosis. In that context, a compound library has been screened in order to identify selective inhibitors to target the activity of the relapse-specific form of the enzyme.
Applications
- Treatment of relapsed ALL – novel inhibitors: Development of a new inhibitor targeting the activity of the NT5C2 enzyme.
- Prevention of relapse: NT5C2 inhibitors could be used as a co-treatment with first-line chemotherapy in patients harboring NT5C2 mutations to prevent relapse.
- Potential treatment for other blood cancers: The target described here is likely to be of interest in other forms of leukemia, such as adult ALL where thiopurines are also used.
Advantages
Targeted Therapy: Lead compounds identified with IC50 in the high nM range.
Intellectual Property
NYU has filed a US patent (US11795511) relating to methods and composition for the prognosis and treatment of relapsed leukemia. NYU has filed a PCT application relating to the composition of matter claims for NT5C2 inhibitors.
-
expand_more mode_edit Authors (1)William Carroll, MD
-
expand_more library_books References (1)
- Meyer, J., Wang, J., Hogan, L. et al. , Relapse-specific mutations in NT5C2 in childhood acute lymphoblastic leukemia
-
expand_more cloud_download Supporting documents (2)Product brochureTargeting mutant NT5C2 in Acute Lymphoblastic Leukemia (ALL).pdfMarketing BriefNYU - Targeting mutant NT5C2 in Acute Lymphoblastic Leukemia - Marketing Brief - CAR01-03.pdf (139 KB)